RINP offers analytical method development, method validation and transfer services for Active Pharmaceutical Ingredients (APIs), Drug Production Formulations, cleaning agents used in manufacturing processes, and raw materials/recipients through a specialized staff of knowledgeable and experienced pharmaceutical scientists.
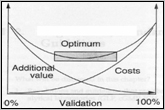
All method validations are performed under cGMP requirements and meet the current ICH and FDA guidelines. In addition, RINP can validate current client methods to help ensure accuracy and reliability of those procedures. The protocol for validations can be client supplied or based on current USP or ICH guidelines. The methods we develop use validation standards that ensure that the developed methods are rugged and robust enough to be used globally. All validation projects can be custom designed to meet your specific analytical requirements.
All our developed methods and results are properly documented in our reports. Reports can be customized to a client's specifications or a standard report can be issued. Standard reports include analytical data, procedure, and supportive documentation such as chromatographs and standards. Our scientists are available to answer any question about a procedure long after the project has been completed. At RINP, we are dedicated to your total satisfaction.
Method Validation Parameters
- Stability Indicating
- Specificity
- Method Precision
- Sample Precision
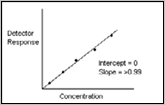
- Linearity and Range
- Accuracy
- LOD/LOQ
- Ruggedness
- Standard and Sample Solution Stability
- Robustness (Optional)
|